- Gases don't have any definite volume and shape.
Compressibility Factor :
The magnitude by which gas deviate from ideal behavior is called Compressibility Factor and is denoted by Z.
Equations for the compressibility factor are:
And,
The compressibility factor can easily be explained by using ration of real and ideal volumes and there are three possibilities.
- If Z=1 then, gas shows perfectly ideal behaviour.
- If Z>1 then, gas show real behaviour with repulsive force dominance.
- If Z<1 then, gas show real behaviour with attractive force dominance.
Effect of Temperature & Pressure on Compressibility Factor :
The effect of temperature and pressure on compressibility factor is totally explained tutorial in the given videos below.
And if you want more illustration you will easily get it from the next video.
Vander Waal's Equation for Real Gas:
Vander Waal's equation proposed that the volume of a gas at real condition is not negligible as compared to the ideal gas and there is intermolecular attractive forces present between them. So, the ideal gas equation should be corrected.
1. Volume Correction:
The volume of ideal gas is smaller than the volume of real gas.
Vi = Vr - nb
Where,
- Vi is the volume of ideal gas
- Vr is the volume of real gas
- n is the number of moles
- b is the effective volume
2. Pressure Correction:
Pressure of real gas is directly proportional to the square of concentration of gas (for one type of gas). If 2 gases are present in container then it is proportional to the concentration of A and B gases.
p = an^2/v^2
Where,
- p is the net attractive pull or force
- a is the attractive force constant
Now the pressure of ideal gas is given by,
Pi = Pr + (an^2/v^2)
Now, the Vander Waal's equation can be written as,
Link between Vander Waal's Equation and the Compressibility Factor:
I. At relatively Low Pressure :
Neglecting Pb with respect to a/v and ab/v2 because lesser pressure, lesser will be effective volume.
Neglecting ab/v2 with respect to a/v because lesser pressure, greater will be the volume. So the factor with v2 in the denominator will be negligible.
II. At very Low Pressure & High Temperature:
Neglecting a/v, Pb, ab/v2 because at low pressure or high temperature, effective volume become smaller and volume become greater.
III. At High Pressure:
Neglecting a/v and ab/v2 with respect to Pb because at high temperature, there will be no attractive forces between molecules.
Andrew Isotherms :
Andrew divided the whole graph of Isotherms of carbon dioxide into three sections.
(a) Isotherm above 31.1C :
Above critical point the curve is a hyperbole and it can easily be seen that with the increase in pressure volume decreases so here Boyle's law hold good.
(b) Isotherm below 31.1C :
Isotherm below critical temperature is further divided into 3 parts:
- The curve AB shows that volume is inversely proportional to the pressure applied. Hence, follow Boyle's law.
- Now the curve BC is a straight line parallel to volume axis. This shows that volume is decreased drastically and the pressure is constant. This concludes that while going from B to C a homogenous mixture of different density substances is formed which has no effect of pressure.
- Now the curve CD is a straight line parallel to pressure axis. This shows that volume becomes constant with the increase in pressure. Pressure has no effect on the volume of formed substance.
(c)
Isotherm at 31.1C :
The curve EF indicates the ideality of gas. The point F is called critical point (at which two states intermingled with each other and we cannot distinguish between the phases. Now when going from F to G, when pressure is increased there is no effect on the volume of formed gas. So it is obvious that a substance is formed which has higher density than gas.
Gas cannot be liquified above critical temperature. Gas can liquefy below and at critical temperature. But below critical temperature, we have to hold the gas molecules close for some time.
Thomson Curve

Thomson said that if a gas can liquefy at critical temperature (without holding) as well as below critical temperature (with compressed holding), then their curve should resembled. He stated that:
"There should be three volumes of gas at every condition of temperature and pressure."
Mathematical Explanation of Thomson Curve by Vander Waal:
Vander Waal used the real gas equation to verify that gases have 3 volumes at every condition of temperature and pressure.
To introduce the concept of cubic volume, he multiply whole equation by v2,
To separate the cubical volume from pressure he divide whole equation by P, we get:
The volume of a gas at critical temperature and pressure is equal to its critical volume.
Specific Heat of Gases :
The amount of heat required to raise a temperature of 1 kg mass of body by 1C or K rise in temperature is called Specific Heat.
The ratio of specific heat at constant pressure and volume is used to explained the nature and atomicity of gases.
𝜸 = f+2/f
Where, f is the degree of freedom or complex motion factor.
Degree Of Freedom:
The degree of freedom is the way a molecule vibrate, rotate or translate in a direction.
- Only diatomic and polyatomic show rotatoryand translatory motion and it can show vibratory motion due to its strong bonding and at very high temperature.
- Whereas, monoatomic molecules could only show translatory motion.
- Complex motion is the sum of all the molecule's rotatory and translatory motion (neglecting exceptional case of vibratory motion).
Total f = f(rotatory) + f(translator)
Note: If anyone find my blog interesting and want numericals of the related topics, he or she will contact me. My whatsapp number is *923150202165.



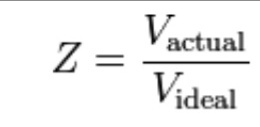




















please send me more information about complex motion factor
ReplyDeletePlease send me ideal gas
ReplyDelete